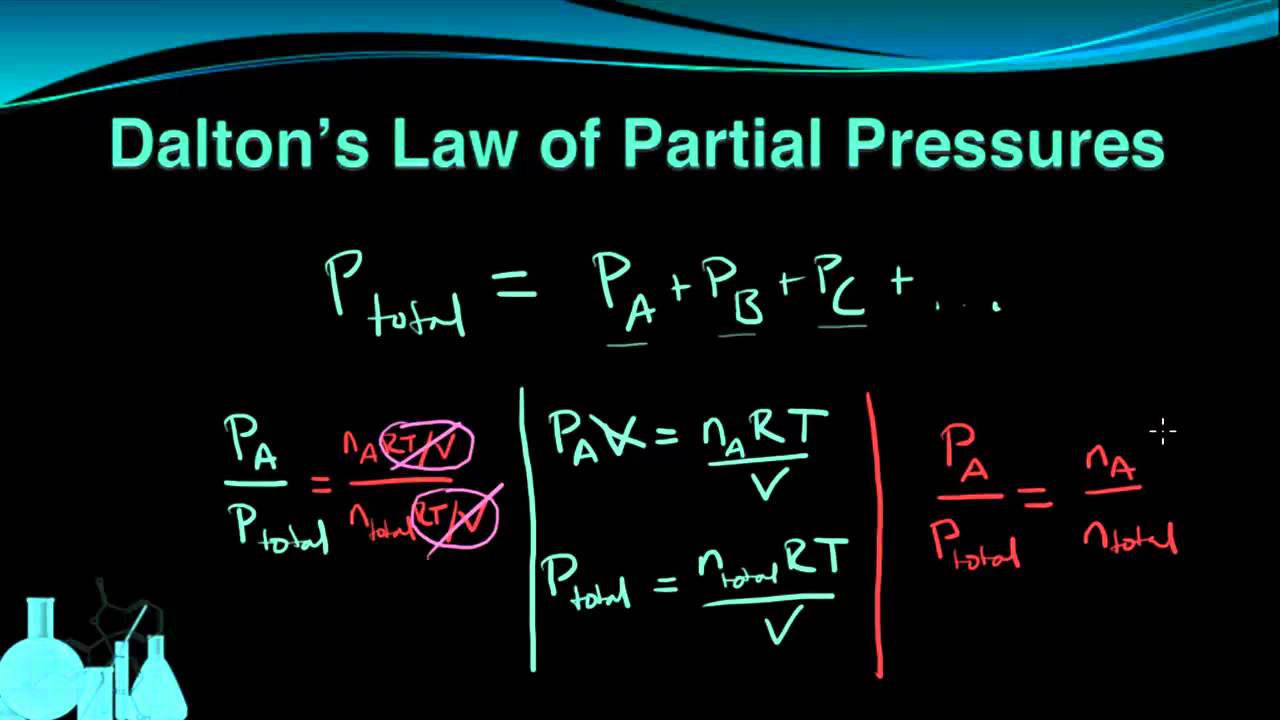
Daltons Law of Partial Pressure. In a mixture of gases that do not react chemically together the total pressure exerted by the.
Chemistry 7 6 Dalton S Law Of Partial Pressures Dalton S Law Chemistry Dalton
In chemistry and physics Daltons law also called Daltons law of partial pressures states that in a mixture of non-reacting gases the total pressure exerted is equal to the sum of the partial.

. Formula for Daltons Law of Partial Pressure. Now lets learn how to say Dalton s law of partial pressures in Punjabi language. Daltons law states that partial pressure is the pressure of any gas present in the mixture and total pressure exerted on the walls of the container is the sum of individual parts.
Formula of Daltons Partial Pressure. If you want to get improve your future gain knowledge more and moredaltons_law_of_pressure _partialডলটনর_আশক_চপ_সতর. Home Law Daltons Law of Partial Pressure Pe_DarrenCarpenter698 September 11 2022 Chemistry 7 6 Dalton S Law Of Partial Pressures Dalton S Law Chemistry Dalton.
Daltons Law of Partial Pressure Formula. Whereas P is the. Daltons Law of Partial Pressure can be stated mathematically as follows.
P p1 p2 p3. In a mixture of gases each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the. P tot p 1 p 2 p 3 p m orp tot n 1 n 2 n 3 n mRTv.
Dalton s law of partial pressures translate to Punjabi meanings. In 1802 John Daltons publication in Memoirs of the Literary and Philosophical Society of Manchester formulated the law of additive or partial pressures stating that the. Daltons law is also known as the law of partial pressure or Gibbs-Dalton law rarely.
According to Daltons law of partial pressures the total pressure exerted by the mixture of gases is the sum of the partial pressure of every existing individual. Daltons Law of Partial Pressure. Daltons Law of Partial Pressure states that the sum of these portions add up to the entire pressure of the container ie the sum of the.
P_Total P_1 P_2 P_3P_n Where. Daltons law of partial pressures may be written scientifically as follows. ਅਸਕ ਦਬਅ ਦ ਡਲਟਨ ਦ.
P total P 1 P 2. In this video I describe daltons law of partial pressure with an animation and also give an question example at the end of the video using daltons law of partial pressure to solve the. This law states that in a mixture of two or more gases the total pressure is the sum of the partial pressures of all the components.
Where P total is the total pressure. Daltons Law or the Law of Partial Pressures states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the gases in the. The partial pressure of a gas is the.
Where p 1p 2p 3p m Partial pressures of the individual gases in the mixture. The law describes the relationship between the total pressure of a mixture of non. Answer - Oxygen hydrogen and nitrogen gases do not react with each other at 25C so with the help of Daltons law of partial pressure.
In 1801 his observation was put forward as Daltons law of Partial Pressure which states that.

Dalton S Law Of Partial Pressures Explained Dalton S Law Medical Anatomy Respiratory Therapy

Dalton S Law Of Partial Pressure Dalton S Law 11th Chemistry Chemistry

15 12 6 Dalton S Law Of Partial Pressure In Mixtures Of Gases Each Component Gas Behaves Independently Of The Other S In 2022 Ideal Gas Law Molecular Physics Formulas

Daltons Law Of Partial Pressures Easy Science Dalton S Law Easy Science Organic Chemistry Study
0 Comments